MaxiNovel Pharmaceuticals Co.,Ltd. is a China based biotech company founded in 2016 to focus on the innovative drug research with the global intellectual property rights.
We carry out first-in-class small molecule drug discovery in the areas of targeted therapy and immunotherapy. Our rapidly expanding product pipeline is mainly composed of programs that target both blood tumors and solid tumors as well as autoimmune diseases. Company’s research platforms encompass oral therapy, radiotherapy, imaging and transdermal therapy.
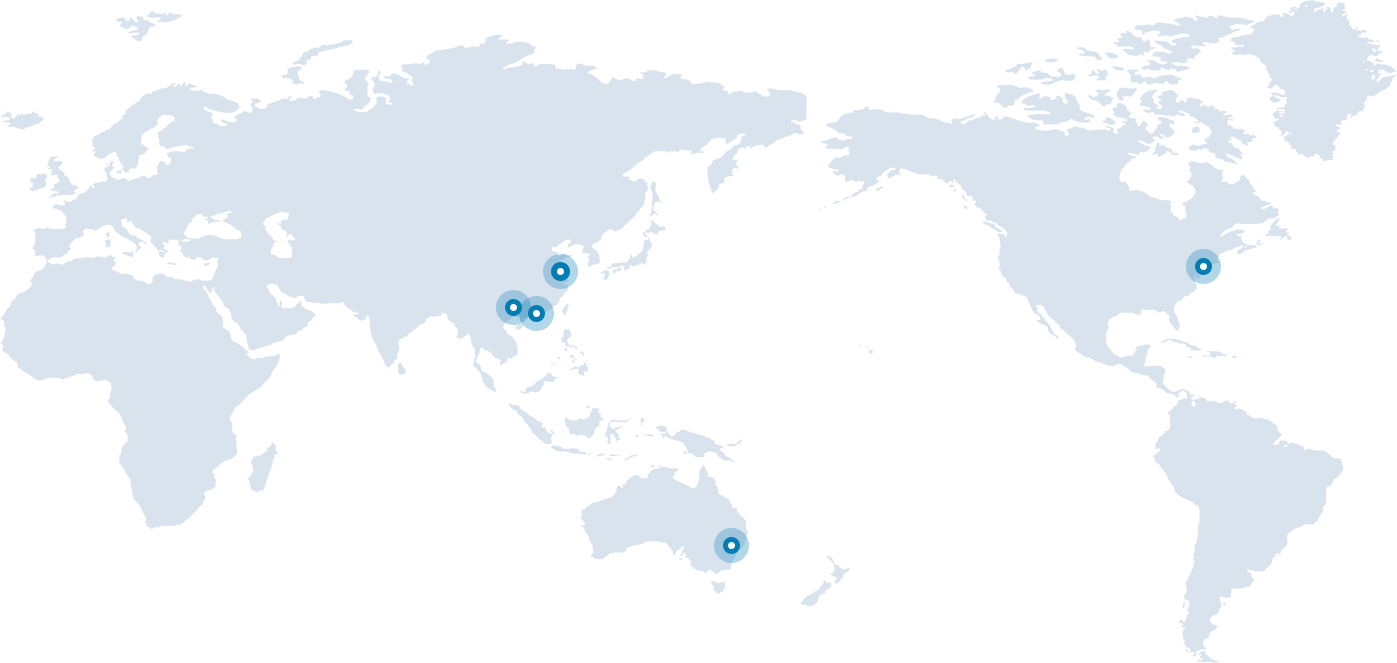
We have our R&D and operation centers in China (Shanghai, Guangzhou, Shenzhen), the United States and Australia. Our management team members all have extensive previous working experiences in global pharmaceutical companies and capital markets, more importantly global vision of our company’s future development.
Our mission is to conduct pharmaceutical innovation to save lives and bring hopes to patients and their families.

Transdermal Drug
Delivery R&D Center
MaxiNovel upholds the people-oriented company philosophy. It motivates employees to help and support each other; encourages everyone to fully utilize one’s creativity and enthusiasm. The company provides a platform for all the employees to realize the company value and personal value at the same time, to share the company success together.

· Over 30 years of Drug R&D and management experiences
· Director of Discovery Collaboration at Schering-Plough Corp
· Inventor of Vorapaxar, an antithrombotic drug approved by FDA
· Recipient of the 2008 Edison Patent Award
· Senior Vice President of ChemPartner
· Key member to drive the listing of ChemPartner’s shares on the New York Stock Exchange (NYSE) in 2010
· PhD in Medicinal Chemistry, University of Kansas, USA.
· Over 15 years of experiences in new drug R&D
· Director of R&D in ShangPharma
· Director of Medicinal Chemistry in Shanghai Explorer
· Group Leader of Medicinal Chemistry in Jiangsu Hengrui Pharmaceuticals, Inc
· PhD in Organic Chemistry from Freie Universit?t Berlin, Germany
· Over 10 years of research and development experience in translational medicine and biomarkers
· Over 5 years of experience in the development of nuclear diagnostic drugs
· Rich research experience in tumor targets and tumor drug mechanisms
· PhD in Biochemistry and Cell Biology, Tongji University
· Postdoctoral fellow, Institute of Biophysics, Chinese Academy of Sciences· Over 10 years of medical experience
· Over 5 years of experience in early stage clinical drug development
· Rich experience in early stage clinical research design, development of new tumor drug research and development pipelines, etc
· Master of Pharmacy, established a medical team to develop new indications for chronic drugs